SCP Tech Brief: Cement Hydration
Hydration, in reference to Portland cement/concrete, is the chemical reaction between cement and water. This process can be observed in 5 stages. Stage 1 begins when water and cement are introduced either by themselves or within a Portland cement concrete mix, and dissolution is initiated. The dissolution stage starts at the plant during the concrete batch mixing. This consists of the surface of the cement particle changing and becoming covered in an early calcium silicate hydrate (C-S-H) gel material. As the cement changes and dissolves, it releases calcium ions in the water, creating pore solution. Stage 2, the induction period, begins after this initial reaction.
At this point very little heat is given off and calcium ions continue to increase. The concrete remains fluid and workable during placement. This period usually lasts about two hours. Eventually, the concentrations increase to the point that the pore solution is supersaturated, meaning that it is energetically favorable for some of the ions to combine into new solid phases rather than remain dissolved1. This is where Stage 3, the rapid reaction period, begins.
This stage typically occurs around two hours after cement is introduced to water and ends around 6 hours2. The time period is variable given the concrete mix design and ambient weather conditions etc. During this stage, hydration products are starting to nucleate and grow. These hydration products, primarily calcium hydroxide (CH) and C-S-H, are different from the starting cement minerals (i.e. C3S, C2S, C3A, C4AF). (See Figure 1 below.)
| Cement Notation | Actual Formula | Name | Mineral Phase | Primary Function |
|---|---|---|---|---|
| C2S | 2CaO · SiO2 | Dicalcium Silicate | Belite | Strength Beyond 7 Days |
| C3A | 3CaO · Al2O3 | Tricalcium Aluminate | Aluminate | Early Strength Development |
| C4AF | 4CaO · Al2O3 · Fe2O3 | Tetracalcium Aluminoferrite | Ferrite | Controls Early Setting, Gives Gray Color |
Stage 3 is largely associated with the hydration of C3S. As more CH and C-S-H precipitate out of the pore solution and grow in size, the hydration rate increases. Precipitation relieves the supersaturation of the pore solution and allows dissolution of the cement minerals to continue. This is a continuous process by which the cement minerals are replaced by new hydration products, with the pore solution acting as a necessary transition zone between the two solid states.
By the end of Stage 3, 30% of the initial cement has hydrated and the paste has gone through initial and final set1. See Figure 1 below.
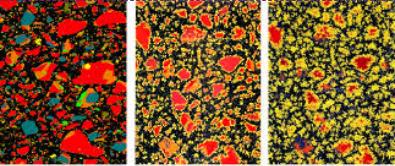
Entering Stage 4, C-S-H becomes thicker on the surface of cement, making it more difficult for water to reach the cement particle. Pore volume decreases, and strength is beginning to develop. This period continues to grow hydration products rapidly up to around 24-30 hours. Beyond 24-30 hours is Stage 5.
During Stage 5, hydration proceeds at a slow rate as long as water is available. In order for further hydration to take place, the dissolved ions from the cement must diffuse outward and precipitate into the capillary pores, or water must diffuse inward to reach the unreacted cement cores. Hydration will cease if the water is removed from the system. This is why proper curing is paramount to the strength and durability of concrete. After 28 days, the cement particles are dominated by C-S-H gel and porosity is noticeably decreased. Typically, about 70% of the cement particles are hydrated after 28 days1.
The most critical time in the life of concrete is the first 24 hours. How concrete is designed, mixed, placed, and most importantly cured has a direct effect on the desired properties of the concrete. Proper curing of concrete consists of providing adequate time, controlling concrete temperature, and maintaining enough moisture to achieve the best results whether the goal is compressive strength or other durability factors. Spray-Lock Concrete Protection (SCP) technology works with the hydration process in the cement to maximize the potential of concrete. SCP time of placement products are applied at the end of Stage 3 in the hydration process. After reaching final set, SCP enters the concrete through capillary pores previously occupied by water. In a completely hydrated cement paste, about 50-60% is occupied by C-S-H, and 15-25% CH3. The colloidal silica in SCP technology reacts with excess Calcium Hydroxide (CH) to create more C-S-H in the concrete, the main hydration product responsible for strength. The accessible capillaries and pores are permanently filled, sealing the concrete and locking in the remaining moisture. By maintaining moisture in the concrete, the hydration process is extended as long as temperature is controlled.
[1] Dr. Hamlin Jennings, The Science of Concrete, Northwestern University, Evanston, IL 2018
[2] Tyler Ley, PH.D, P.E., Portland Cement Hydration, Oklahoma State University, Stillwater, OK 2019
[3] Kosmatka, S.H.; Wilson, M.L. Design and Control of Concrete Mixtures, 15th edition. Portland Cement Association, Skokie, IL, USA 2011 460pp.
[Fig 1] Figure 1 illustrates the microstructure of cement paste as it hydrates. Notice the growth of C-S-H on the cement minerals over time. The blue is C2S, the red is C3S, black is pore space, and yellow is C-S-H1.

